Shanghai chengsi intelligent technology co., LTD
Contact: Mr. Gao
Phone: 17701637746
Phone: 19821311892
Address: no. 265, east street, zhuangxing town, fengxian district, Shanghai


Plastic handle scalpel assembly firmness tester
According to the YY0454 standard
1. qualification:
Force value measurement range: 0~80N, resolution: 0.01N, precision: ± 1%;
Test time: 1S to 59MIN adjustment
Fixed block span: 0~150MM arbitrary adjustment
2. scope
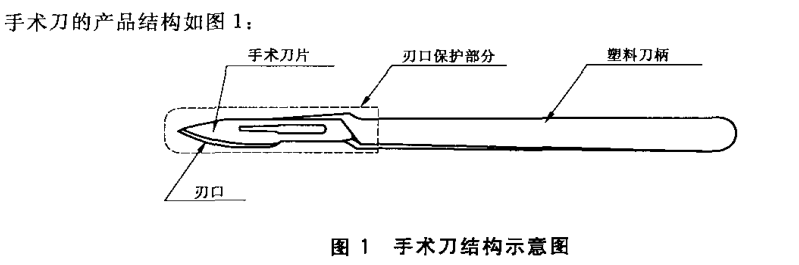
This standard specifies the structure and marking, requirements, test methods, inspection rules, marking, packaging, transportation, storage and service life of the sterile plastic handle scalpel.
This standard is applicable to the sterilized packaging of disposable sterile plastic handle scalpel (hereinafter referred to as the scalpel), which is used for soft tissue cutting during surgery or dissection.

3. Normative reference documents
The terms in the following documents become the provisions of this standard by reference to this standard.All subsequent amendment orders (excluding errata) or amendments do not apply to this Standard, however, the parties to an agreement under this standard are encouraged to study the availability of the latest versions of these documents.The latest version applies to this standard.
GB / T 1220 2007 stainless steel bar
GB / T 1298- -1986 Carbon tool steel technical conditions
GB / T 12992000 alloy tool steel
GB / T 2828.2003 Count Sampling Inspection Procedure-Part 1: Receiving Quality Limit (AQL) (ISO 2859-1:1999, IDT)
GB/ T 1 6886. 7
2001 Biological evaluation of medical devices — Part 7: sterilization residue of ethylene oxide (idt
ISO 10993—7:1995)
YY / T 01 71 2008 Surgical device packaging, marking, and use instructions
YY 0174- -2005 surgical blade
YY / T 0466-2003 Medical device symbols for labeling, marking, and providing information (
ISO 15223:2000,IDT)
Secidity test of blade and handle
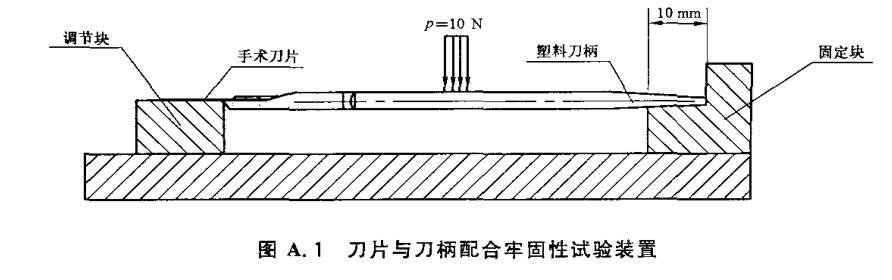
4. Unit requirements
The upper plane of the support blocks (fixed and regulator blocks) supporting both ends of the scalpel shall be maintained at the same plane.
The left and right direction of the adjustment block edge (as shown) shall be adjustable, and the adjustment range shall meet the length requirements of different specifications.
Loading should be at a uniform speed, without impact.
5. experimental method
Remove (or remove) the blade shield (or sleeve) of the scalpel.
Adjust the position of the adjustment block to the corresponding scalpel.
Place the scalpel in the illustrated position to contact the plane of the surgical blade with the upper plane of the adjustment block.
No impact plus load of 10N in the middle of the plastic handle, maintained for 30s.
Remove the load and observe that the surgical blade shall not fall off and have no obvious loosening.
Shanghai chengsi intelligent technology co., LTD
Contact: Mr. Gao
Phone: 17701637746
Phone: 19821311892
Address: no. 265, east street, zhuangxing town, fengxian district, Shanghai
